The State of Zinc in Methanol Synthesis over a Zn/ZnO/Cu(211) Model Catalyst
Result of the Month
In this work, we demonstrate that a specially engineered ambient-pressure XPS experiment, based on a design with local high pressure and extreme grazing incidence of incoming x-rays, is capable of providing high surface sensitivity. This approach enables investigation of the nature of Zn and surface-adsorbed intermediates with a pressure of several hundred millibar at elevated temperatures, thereby shifting toward more-realistic conditions for methanol synthesis. To enable a direct comparison with theoretical calculations that aimed to describe the industrial catalytic process, we selected an identical model system with a stepped Cu(211) single crystal promoted by Zn. This system exhibits superior turnover frequency relative to other more-compact surfaces such as Cu(111) and Cu(100).
From Zn 3d core-level shifts, we found that the nature of Zn depended on the reaction gas mixture. Incidence angle–dependent spectra showed that, in CO2-rich conditions, ZnO was favored to exist as bulk-like particles, whereas CO tended to generate metallic Zn that was alloyed with the Cu near the surface. From the C 1s spectra, we concluded that for CO2 reduction there was a codominance of formate and methoxy as long-lived intermediates, whereas in CO the methoxy species dominated. The coverage of the intermediates diminished at higher temperature, indicating that the reaction was turning over. The aforementioned results support a model in which higher methanol production yield with a mixture of CO2 and CO is a consequence of the strong reducing ability of CO, which generates a surface that has a high density of alloyed Cu-Zn sites and is particularly active for CO2 reduction to methanol.
Schematic of the experiment and x-ray photoelectron spectra under different gas environments.
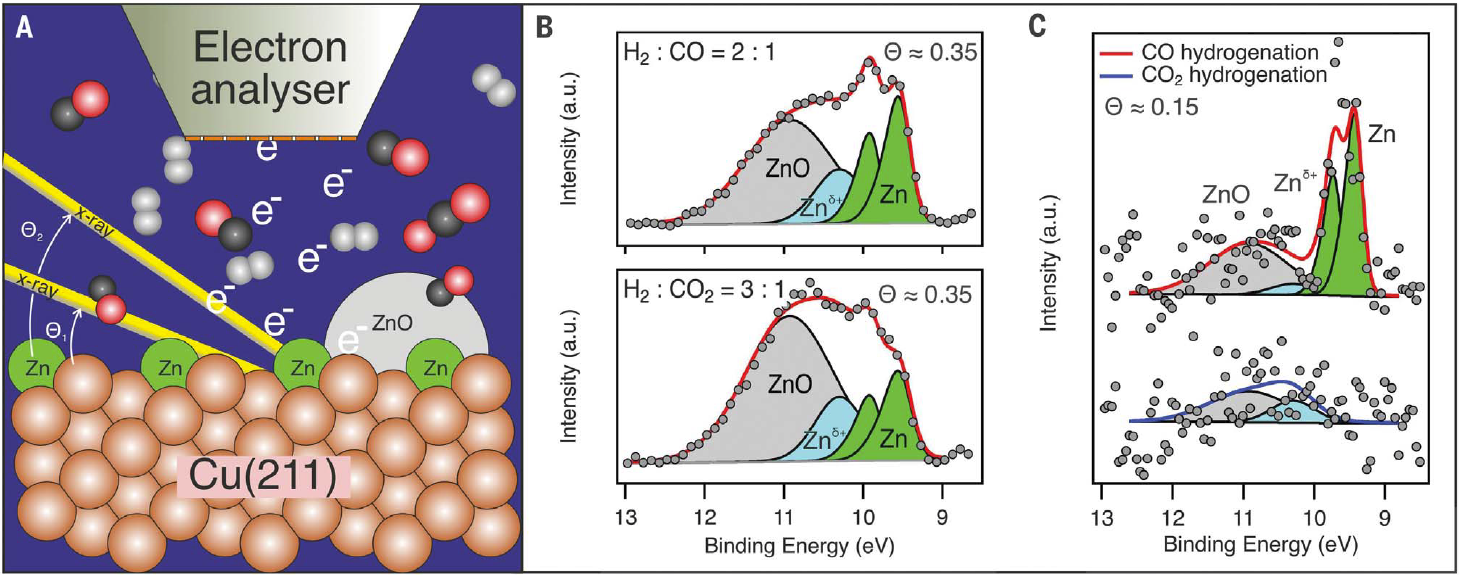
Figure Description: (A) The surface of Zn/ZnO/Cu(211) is probed with grazing incidence x-rays at different angles (Q1 and Q2) while the surface is exposed to an elevated pressure of a gas mixture of CO, CO2, and H2. The surface was heated from the backside to achieve reaction conditions. (B) Experimental x-ray photoelectron spectra of the Zn 3d region obtained at 180 mbar, 140°C, and stoichiometric gas composition, using 4750 eV of photon energy. The experiment was conducted at ~35% surface Zn. The relative amount of ZnO to metallic Zn shows a strong dependency on gas composition. a.u., arbitrary units. (C) Experimental x-ray photoelectron spectra of the Zn 3d region obtained at 500 mbar, 230°C, and H2:CO = 2.6:1 and H2:CO2 = 2.6:1, using 4600 eV of photon energy at a Zn coverage of ~15%. The spectra contain more noise because of the elevated pressure but exhibit more-pronounced differences between the two gas compositions.
Experiment
The Zn/ZnO/Cu(211) surface was prepared by evaporating metallic Zn and subsequent thermal annealing inside the vacuum chamber without exposure to air. The sample surface was then placed parallel to and within 30 mm of the electron spectrometer entrance. Precleaned and premixed gases of CO, CO2, and H2 were directed onto the sample surface, which created a localized volume of elevated pressure that acted as a small virtual cell with rapid gas flow. Using a well-focused and low-divergence x-ray beam from beamline P22 at the Petra III synchrotron radiation facility, we probed the surface under grazing incidence conditions with a precision of ±2 mrad.
-
Authors:
Peter Amann1†, Bernhard Klötzer2, David Degerman1, Norbert Köpfle2‡, Thomas Götsch3, Patrick Lömker4,1, Christoph Rameshan5, Kevin Ploner2, Djuro Bikaljevic2, Hsin-Yi Wang1, Markus Soldemo1§, Mikhail Shipilin1, Christopher M. Goodwin1, Jörgen Gladh1§, Joakim H. Stenlid1¶, Mia Börner1, Christoph Schlueter4, Anders Nilsson1*
Institutes:
1) Department of Physics, Stockholm University, AlbaNova University Center, 10691 Stockholm, Sweden.
2) Institute of Physical Chemistry, University of Innsbruck, Innrain 52c, 6020 Innsbruck, Austria.
3) Department of Inorganic Chemistry, Fritz Haber Institute of the Max-Planck Society, Faradayweg 4-6, 14195 Berlin, Germany.
4) Photon Science, Deutsches Elektronen-Synchrotron DESY, Notkestr. 85, 22607 Hamburg, Germany. 5Institute of Materials Chemistry, Technische Universität Wien, Getreidemarkt 9/BC/01, 1060 Vienna, Austria.
†Present address: Scienta Omicron AB, Danmarksgatan 22, 75323 Uppsala, Sweden.
‡Present address: Plansee SE, Metallwerk-Planseestraße 71, 6600 Reutte, Austria.
§Present address: PULSE Institute, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, CA 94025, USA.
¶Present address: SUNCAT Center for Interface Science and Catalysis, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, CA 94025, USA.
Instrument:
HiPP-2 Analyser integrated into BAR XPS endstation