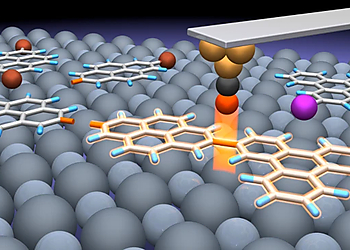
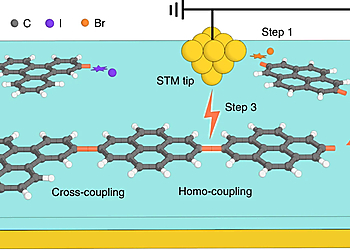
Constructing Covalent Organic Nanoarchitectures Molecule by Molecule via Scanning Probe Manipulation
Result of the Month
In this study, we revisit Feynman’s and Drexler’s visions and utilize the STM tip and the terraces of NaCl thin films as ‘non-fat and non-sticky fingers’ for covalently connecting different molecular building blocks via controlled manipulations (Fig. 1). The outcome of the sequential reaction steps can be conveniently followed by visual inspection with high-resolution low-temperature AFM with CO-functionalized tips, which allows the identification of the chemical structures of the precursors, intermediates and products.
The approach enables the bottom-up engineering of elusive organic nanoarchitectures with atomic precision. Additionally, it allows studying the pathways of intermolecular reactions between aromatic carbon radicals by molecular manipulation. Ultimately, it paves the way for systematically studying the effects of structural modifications on the properties of organic materials, which is important for understanding and controlling molecular functionality.
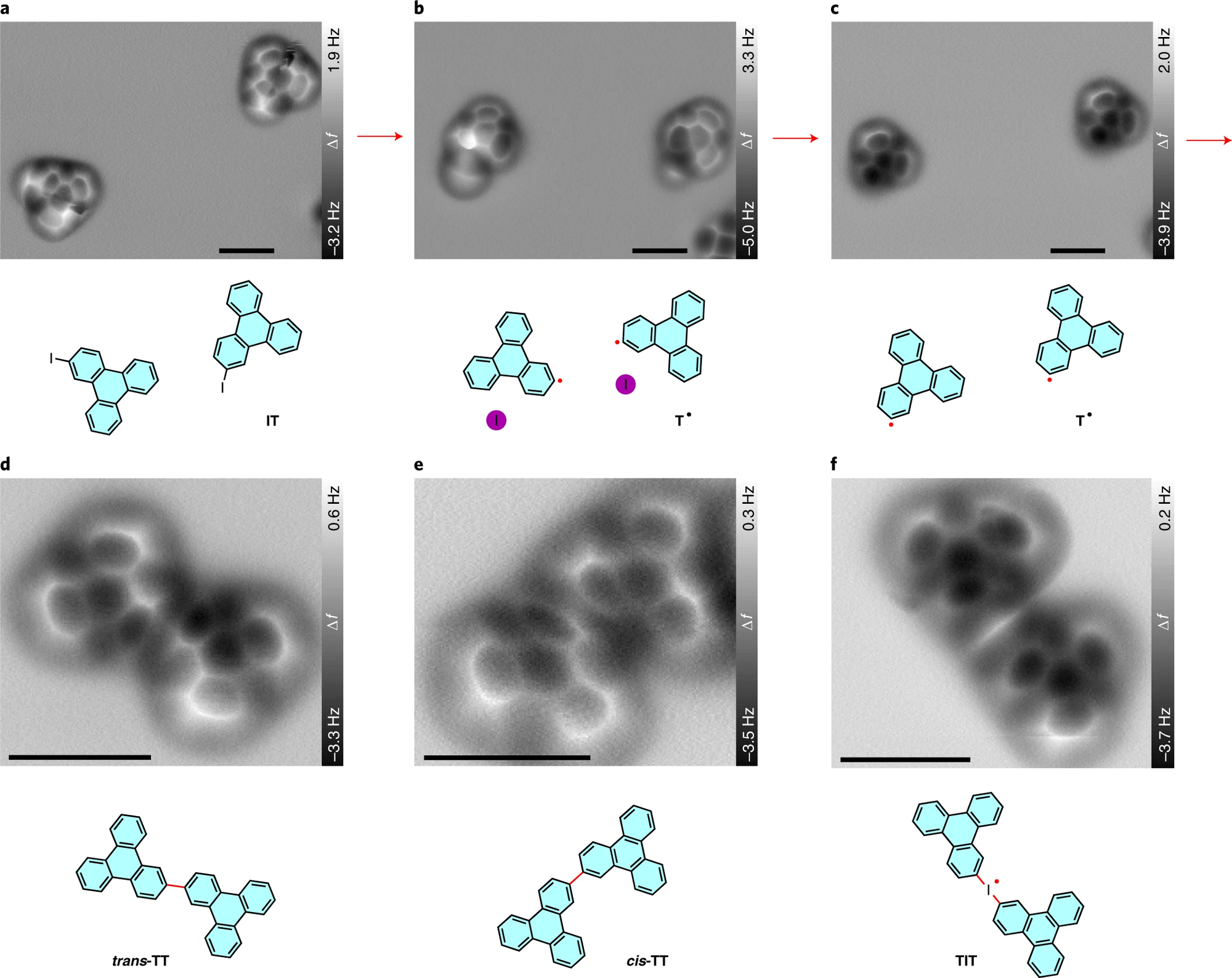
Figure Description: Tip-induced deiodination and intermolecular homo-coupling of IT on NaCl(2 ML)/Cu(111).
a–c, Constant-height AFM frequency shift (Δf) images of two pristine IT molecules (a) and the adsorbed T* radicals before (b) and after (c) removing the adjacent iodine atoms. d,e, AFM images of a trans isomer (d) and a cis isomer (e) of TT produced by tip-induced homo-coupling of two T• radicals. f, AFM image of an iodine-bridged triphenylene dimer TIT. Chemical structures are presented below the corresponding AFM images. The newly formed radicals and bonds are indicated by red dots and lines, respectively. The images are collected from different series.
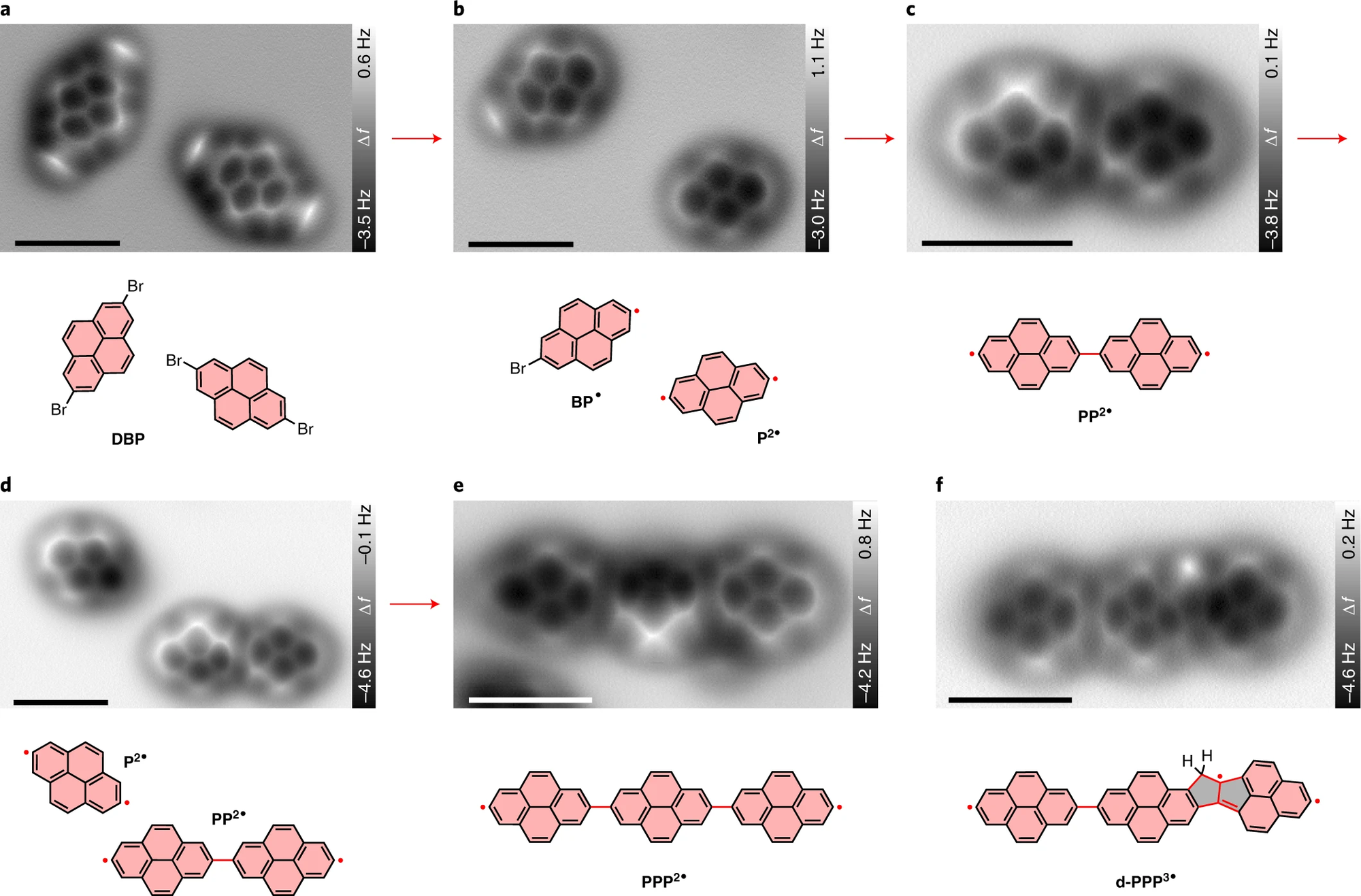
Figure Description: Tip-induced debrominative homo-coupling and oligomerization of DBP on NaCl(2 ML)/Cu(111).
A few representative AFM images illustrate the main steps for the manipulation. a, Two isolated DBP molecules. b, A BP• monoradical and a P2• diradical. c, A PP2• homo-dimer formed via homo-coupling of two pyrene diradicals. d, A P2• diradical and a PP2• homo-dimer. e, A PPP2• homo-trimer. f, A defective pyrene trimer d-PPP3• with two fused pentagons generated by skeletal rearrangement. Chemical structures are presented below the corresponding AFM images. The newly formed radicals and bonds are indicated by red dots and lines, respectively.
STM and AFM Measurements
All the STM and AFM measurements were performed at ~5.2 K under ultra-high-vacuum conditions (base pressure <1.0 × 10−10 mbar) using a commercial STM/AFM system (Scienta Omicron). The STM tip was grounded, and all the voltages mentioned in this paper refer to the sample bias voltage. We used a qPlus-type force sensor with a resonance frequency of f ≈ 26.98 kHz, an oscillation amplitude of A ≈ 56–71 pm and a quality factor of Q ≈ 10,000–50,000. A small sample bias voltage (submillivolt) was applied during constant-height AFM imaging to minimize the tunnelling current. The positive and negative signs of the tip–substrate distance offset Δz represent the increase and decrease of the tip–substrate distance with respect to a certain STM set point, respectively. CO-terminated tips were exploited for chemical bond resolution. To obtain a CO-terminated tip, a metal tip was first sharpened by several voltage pulses and controlled indentations into the Cu(111) surface, and then the tip was vertically pressed against a CO molecule adsorbed on the NaCl(2 ML)/Cu(111) surface to pick it up. Therefore, the tip was first placed above the CO with an STM set point (typically 500 mV and 2 pA) and then moved towards the CO molecule until a sudden jump in the current signal was observed. The pickup was confirmed by the enhanced contrast of the subsequent STM and AFM images. Iodine atoms were vertically manipulated in a similar way.
Authors:
Qigang Zhong, Alexander Ihle, Sebastian Ahles, Hermann A. Wegner, Andre Schirmeisen & Daniel Ebeling
Institutes:
1. Institute of Applied Physics, Justus Liebig University Giessen, Giessen, Germany
Qigang Zhong, Alexander Ihle, Andre Schirmeisen & Daniel Ebeling
2. Center for Materials Research, Justus Liebig University Giessen, Giessen, Germany
Qigang Zhong, Alexander Ihle, Sebastian Ahles, Hermann A. Wegner, Andre Schirmeisen & Daniel Ebeling
3. Institute of Organic Chemistry, Justus Liebig University Giessen, Giessen, Germany
Sebastian Ahles & Hermann A. Wegner
Corresponding Authors:
- Qigang Zhong: Qigang.Zhong@ap.physik.uni-giessen.de
- André Schirmeisen: Andre.Schirmeisen@ap.physik.uni-giessen.de
- Daniel Ebeling: Daniel.Ebeling@ap.physik.uni-giessen.de
Publication:
Zhong, Q., Ihle, A., Ahles, S. et al. Constructing covalent organic nanoarchitectures molecule by molecule via scanning probe manipulation. Nat. Chem. 13, 1133–1139 (2021). https://doi.org/10.1038/s41557-021-00773-4