Atomic Insight into the Interfacial Effect on the Molecular Solvation
Result of the Month
Scanning tunneling microscopy (STM) has been applied to investigate the microsolvation and hydration processes of molecules, such as small adsorbates, self-assembled organic molecules and biomolecules on solid surfaces. However, it remains difficult to resolve the detailed chemical structure of the complex molecular hydrates, since STM is only sensitive to the electronic states not the position of the atoms. Recently, qPlus-based noncontact atomic force microscopy (nc-AFM) has been applied to visualize the chemical structure of organic molecules and water molecules with single-bond resolution by probing Pauli repulsive forces. Even for the weakly bonded water clusters and ion hydrates, nc-AFM can discern the subtle difference of O−H tilting by probing the weak high-order electrostatic force in a nearly noninvasive manner.
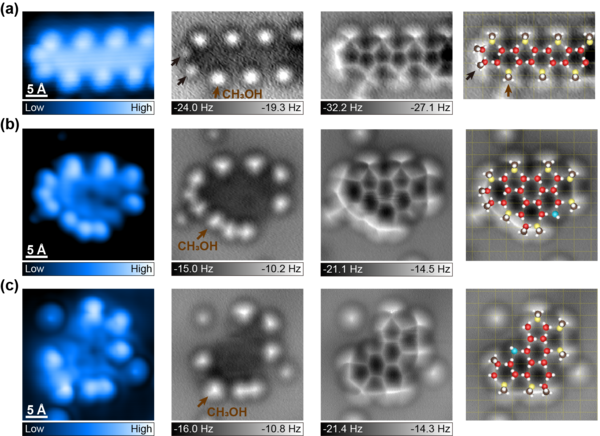
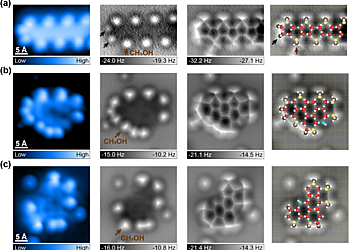
Figure 2. Atomic-scale characterization of water−methanol complexes on Cu(110) with the methanol−water ratio of 2:3. (a−c) High-resolution STM topographies (the leftmost column), constant-height AFM images (the middle two columns) and the proposed atomic models overlaid on the AFM images (the rightmost column). (a) 1D water−methanol chain. (b,c) Water−methanol mixed clusters composed of 15-D2O + 10-CH3OH (b) and 14-D2O + 7-CH3OH (c), respectively. H and C atoms are denoted as white and brown spheres, respectively. Red, yellow, and blue spheres represent O atoms in the flat, vertical water (methanol) molecules and hydroxyls, respectively. Brown and black arrows show methanol molecules with vertical and horizontal OH directionality, respectively, with respect to the surface. The yellow grids indicate the underlying Cu(110) lattice. The set point of STM images: (a) −50 mV, 50 pA; (b,c) 100 mV, 50 pA. Experimental AFM images (the middle two columns) from left to right are obtained at the tip height of 70, −100 pm (a), and 0, −100 pm (b,c), respectively.
In this work, we probe the microsolvation of methanol in water on copper surfaces, using qPlus-based nc-AFM with a CO-terminated tip at 4.8 K. The configurations of water and methanol molecules in the complexes, including the detailed OH directionalities and H-bonding networks could be identified unambiguously by the height-dependent AFM images in combination with theoretical simulations. We demonstrate the excellent cooperativity between the water and methanol molecules through hydrogen-bonding in the formation of ordered clusters on both Cu(110) and Cu(111) surfaces, which are composed of pentagonal and hexagonal rings. Furthermore, we find that the micro-miscibility of methanol and water could be tuned by the substrates. Water and methanol molecules are molecularly segregated and incompletely mixed on the Cu(110) surface. In contrast, the Cu(111) surface would facilitate the accommodation of methanol-containing hydrophobic headgroup within the compact hexagonal and pentagonal water H-bonding network. Density functional theory (DFT) calculations reveal that the energy gain of intermolecular interaction induced by the collective H-bonding network relaxation almost compensates for the binding energy loss between the water−methanol complex and the Cu(111) substrate, thus resulting in the molecular-scale complete mixing of water and methanol.
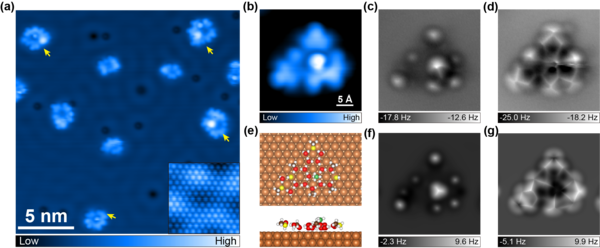
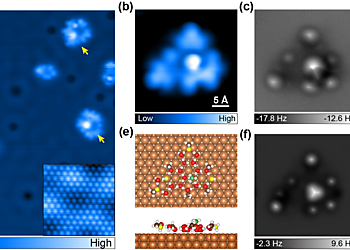
Figure 3. Water−methanol complexes on the Cu(111) surface with the methanol−water ratio of 1:2. (a) Overview STM image (set point: 100 mV, 50 pA). Inset: atomic-resolution STM image of the Cu(111) surface (set point: 10 mV, 5 nA; image size: 3 × 3 nm). Yellow arrows denote water−methanol mixed clusters with interior methanol molecules. (b−g) The detailed atomic characterization of the cluster with a methanol molecule residing at the internal hexagon (14-D2O + 7-CH3OH). (b) High-resolution STM image (set point: 100 mV, 50 pA). (c,d) Experimental AFM images at the tip height of 50 pm (c), −80 pm (d). (e) Top and side views of the calculated adsorption configuration. Green spheres represent C atoms in the methanol molecule residing at the internal H-bonding network. (f,g) Simulated AFM images at the tip height of 11.8 Å (f), 10.2 Å (g).
STM and AFM Experiments
All the experiments were carried out with an ultra-high vacuum Scienta Omicron POLAR-STM/AFM combined system operated at 4.8 K using a qPlus sensor equipped with a W tip (resonance frequency f0= 26.0 kHz, spring constant k0 ≈ 1.8 kN m−1, quality factor Q ≈ 50,000). The Cu(110) and Cu(111) single crystals were cleaned by several cycles of 1.0 keV Ar+ sputtering followed by annealing at 800 and 830 K for 10 min, respectively.
---
Authors: Jia Dong, Pu Yang, Chen Zhang, Duanyun Cao, Ying Jiang, and Jing Guo
Institutes:
Duanyun Cao − Beijing Key Laboratory of Environmental Science and Engineering, School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China; Beijing Institute of Technology Chongqing Innovation Center, Chongqing 401120, China
Jing Guo − College of Chemistry, Key Laboratory of Theoretical and Computational Photochemistry, Beijing Normal University, Beijing 100875, China; Interdisciplinary Institute of Light-Element Quantum Materials and Research Center for Light-Element Advanced Materials, Peking University, Beijing 100871, China
Jia Dong − College of Chemistry, Key Laboratory of Theoretical and Computational Photochemistry, Beijing Normal University, Beijing 100875, China
Pu Yang − College of Chemistry, Key Laboratory of Theoretical and Computational Photochemistry, Beijing Normal University, Beijing 100875, China
Chen Zhang − College of Chemistry, Key Laboratory of Theoretical and Computational Photochemistry, Beijing Normal University, Beijing 100875, China
Ying Jiang − International Center for Quantum Materials, School of Physics and Interdisciplinary Institute of Light-Element Quantum Materials and Research Center for Light-Element Advanced Materials, Peking University, Beijing 100871, China
Name and Email of Corresponding Authors:
Duanyun Cao − Beijing Key Laboratory of Environmental Science and Engineering, School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China; Beijing Institute of Technology Chongqing Innovation Center, Chongqing 401120, China; Email: dycao@bit.edu.cn
Jing Guo − College of Chemistry, Key Laboratory of Theoretical and Computational Photochemistry, Beijing Normal University, Beijing 100875, China; Interdisciplinary Institute of Light-Element Quantum Materials and Research Center for Light-Element Advanced Materials, Peking University, Beijing 100871, China; Email: jguo1294@bnu.edu.cn
Publication: J. Phys. Chem. C 2022, 126, 7, 3756–3763. https://doi.org/10.1021/acs.jpcc.1c10296