Influence of hydrogen-bonded 3-mercaptopropionic acid layers on binary self-assembled monolayer formation
Result of the Month
The influence of the monolayer order, defect density, and bilayer formation on the formation of binary self-assembled monolayers (SAMs) was investigated via the solution-phase displacement of 3-mercaptopropionic acid (MPA) by 1-decanethiol (DT). The ultrahigh vacuum scanning tunnelling microscopy results confirm that well-ordered, pristine MPA SAMs are displaced at slower rates than MPA SAMs with less long-range order and greater defect density. Furthermore, MPA samples containing regions of an MPA bilayer displayed the slowest rates of displacement with DT. For pristine MPA samples and MPA samples with regions of an MPA bilayer, displacement with DT resulted in the formation of the low-density, lying down phase of DT. Our results suggest that the presence of an MPA bilayer, the result of hydrogen bonding between carboxylic acid groups in MPA, significantly lowers the rate of total displacement of MPA by DT compared to moderately defected MPA SAMs. These results highlight the importance of the structure, composition, and intermolecular forces, such as hydrogen bonding, when considering binary SAM formation via solution-phase displacement methods.
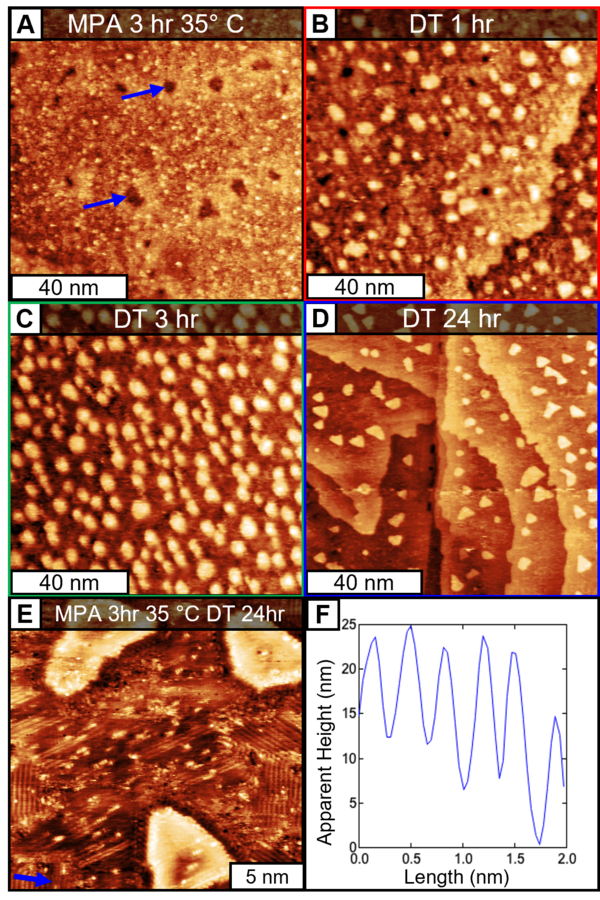
100 nm x 100 nm STM scans of (A) Au/mica immersed in MPA for 3 hours at 35 °C (It = 70 pA, Vt = 150 mV). Representative etch pits are indicated with blue arrows. The sample was subsequently incubated in DT for (B) 1 hr (It = 150 pA, Vt = 450 mV), (C) 3 hr (It = 200 pA, Vt = 300 mV) , (D) 24 hr (It = 180 pA, Vt = 250 mV). (E) 20 nm x 20 nm (It = 110 pA, Vt = 220 mV) STM scan of Au/mica immersed in MPA for 3 hours at 35 °C and subsequently incubated in DT for 24 hr showing domains of the lying down phase of DT. (F) height profile along blue line in (E) verifying the 11.5a periodicity.