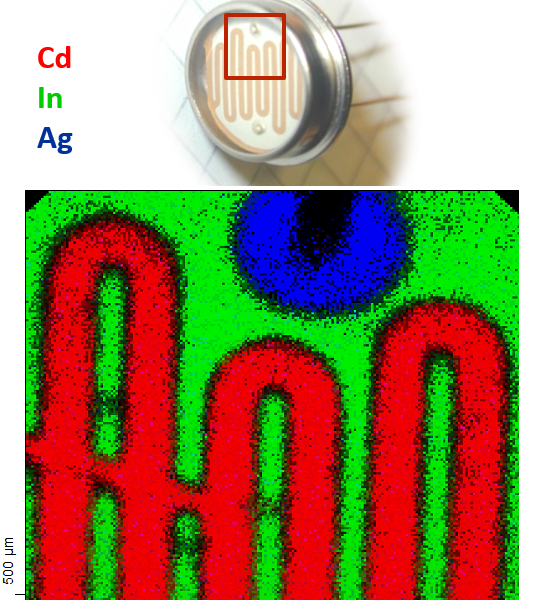
Imaging X-Ray Photoelectron Spectroscopy
X-ray photoelectrons spectroscopy (XPS), also known as electron spectroscopy for chemical analysis (ESCA) is a surface sensitive spectroscopy that allows to determine the chemical composition of surface layers of solid samples. The samples are exposed to X-rays (typically AlKa or MgKa with 1486 eV and 1256 eV photon energy, respectively) under ultra-high vacuum (UHV) conditions. The X-rays excite electrons from the strongly bound core levels in the sample, creating photoions and photoelectrons. The photoelectrons travel through the solid and quickly interact with the surrounding material, thereby loosing their initial kinetic energy. However, a certain fraction (the photoelectrons that have been created close to or at the surface) can escape undisturbed through the sample surface into the vacuum above the sample. There they can be collected and analyzed in terms of their numbers at specific kinetic energies, typically with electrostatic lenses and hemispherical analyzers. Because each material is associated with photoelectrons of different kinetic energies, the resulting spectra can be used to identify and quantify the elements that are present in the near-surface region of the sample. Although the spectrometers usually measure the kinetic energy, it is common practice to plot the spectra with respect to the electrons binding energies. Those energies can be calculated from the measured kinetic energies due to energy conservation and the knowledge of the applied photon energy.
If the experimental setup allows to (i) restrict the origin of the photoelectrons to a certain point on the sample and (ii) collect spectra systematically from different spots on the sample, it is possible to combine these spectroscopic data into a real space spectroscopic image of the surface: imaging XPS. The spectral intensity of a given element can be plotted as brightness (colour-code) as a function of geometrical origin on the sample.