The Donnan potential revealed
Result of the Month
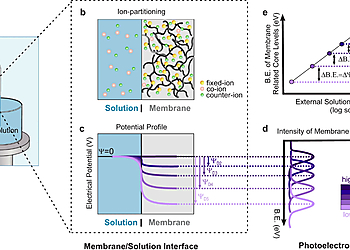
Despite the long history and widespread use of ion-exchange membranes in water and energy applications, direct measurement of the Donnan potential and experimental verification of Donnan’s framework has never been accomplished since the original theory was proposed. We therefore sought to develop an experimental methodology that could directly measure the Donnan potential in a commercial ion-exchange membrane equilibrated with an aqueous salt solution under equilibrium conditions. In this work, we used the “dip and pull” method to form a thin solution layer on the membrane surface. Tender ambient pressure X-ray photoelectron spectroscopy (tender-APXPS) measurements at the solution/IEM interface were performed using a photon energy of 4.0 keV. Our measurements are based on the ability of XPS to detect local potentials through binding energy shift in membrane related core levels.
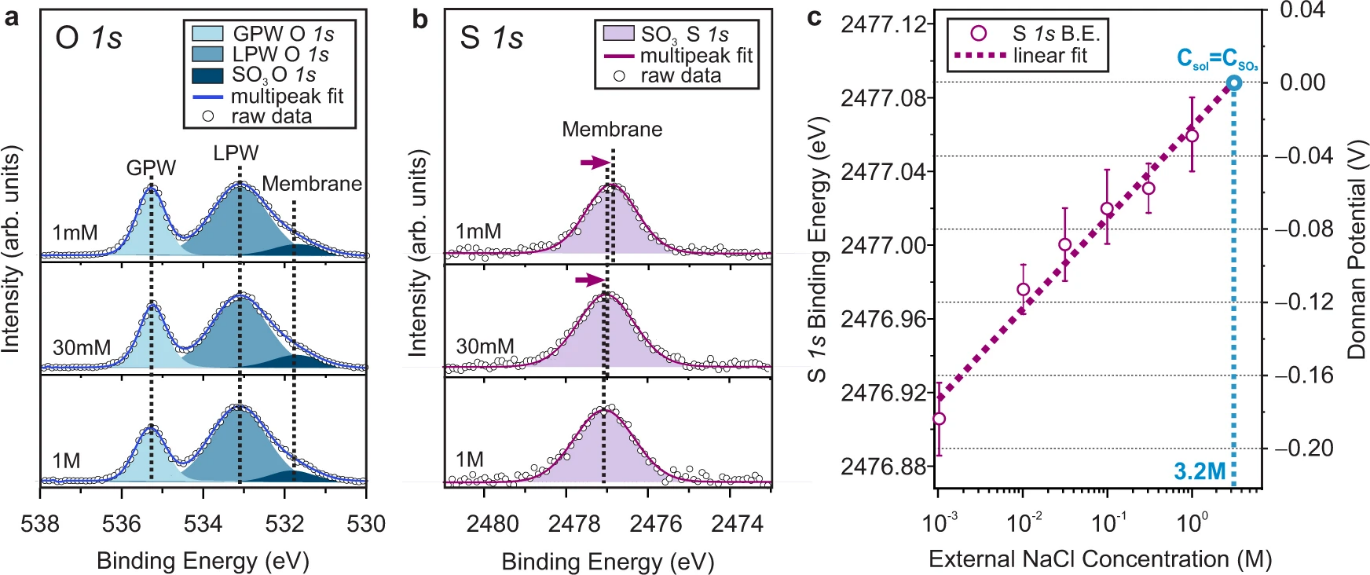
Representative a) O 1s and b) S 1s core level spectra collected from CR-61 membranes equilibrated with different concentrations of NaCl solutions (GPW: gas phase water, LPW: liquid phase water). The binding energy is calibrated using bulk liquid phase water. The effects of the electrical double layer on the binding energy position of the LPW peak are considered, and corrections are made during the calibration process. (Details of the energy calibration process are set forth in Supplementary Note 4) Circles represent raw experimental data, and lines indicate the sum of fits. Representative spectra not provided here for three other concentrations of NaCl solutions are presented in Supplementary Fig. 6 for visual inspection. c Averaged S 1s binding energy and the corresponding Donnan potential values as a function of the external solution concentration. Note that the S 1s binding energy shift versus Donnan potential shows a linear dependence of ∼1 eV/V. Error bars that represent the experimental uncertainty in S 1s binding energy were determined from the standard deviation of repeated measurements of a minimum of five different locations in each case. The binding energy values of fitted core level components for each individual analysis position are provided in Supplementary Table 6. The dashed line represents the best fit to a linear dependence. The fitted line was extrapolated to the equivalent concentration of counter-ions (3.2 M), where the Donnan potential approaches 0 V.
The measurements were carried out in the tender APXPS Beamline, located at Lawrence Berkley National Laboratory’s Advanced Light Source. All core level spectra reported in this piece were taken using 4.0 keV photon energy in normal emission utilizing a a R4000 HiPP-2, from Scienta Omicron and using 100 eVpass energy. With 4.0 keV photon energy, the inelastic mean free path (IMFP; λ) of the O 1s core level corresponds to 9.5 nm, which makes the XPS probing depth ~28.5 nm (from 3λ). During APXPS measurements, pressure in the analysis chamber ranged from 14 to 18 Torr, while the analyser was kept under high vacuum conditions (~4 × 10–7 Torr).
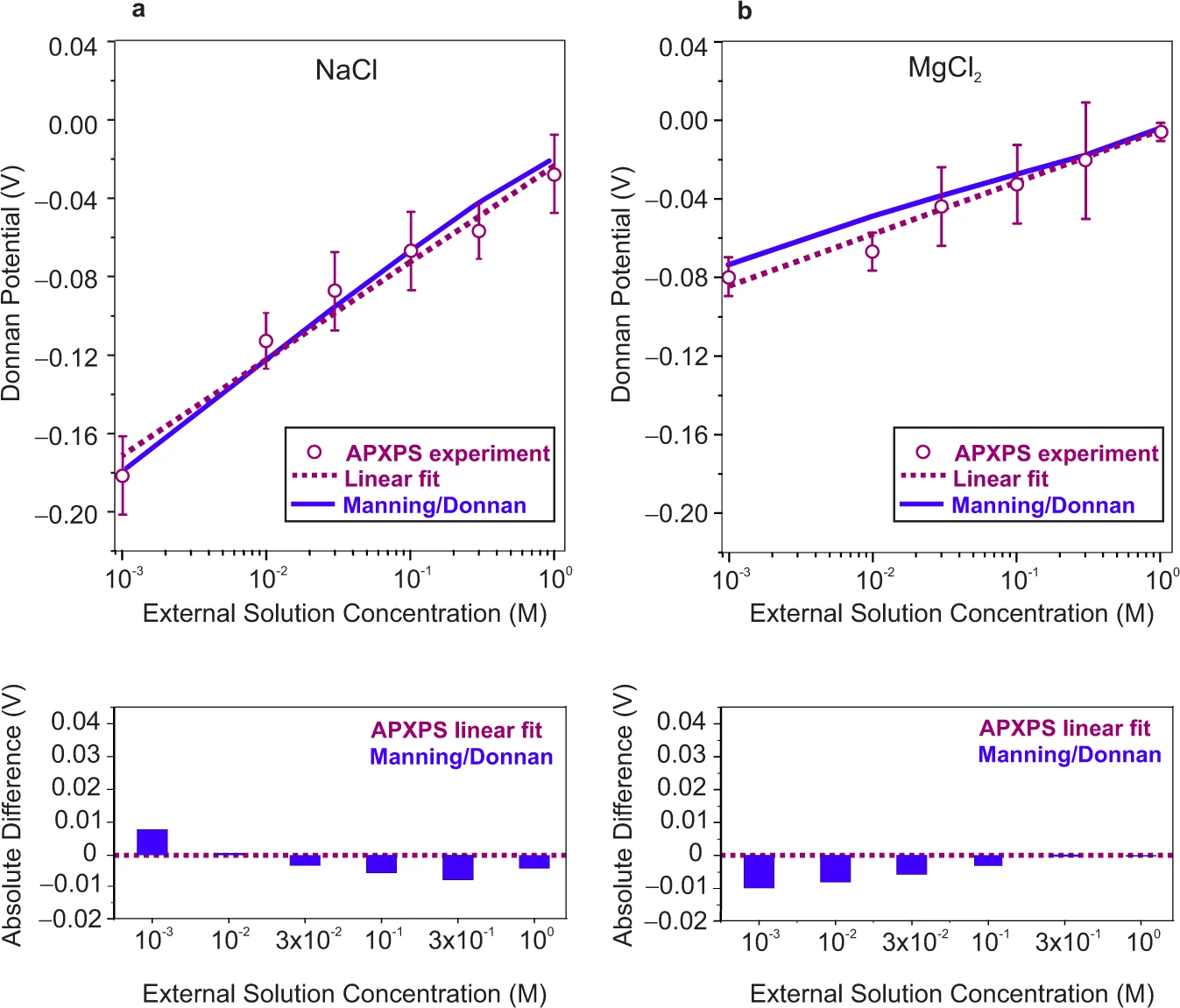
Comparison of experimentally measured Donnan potential values obtained from binding energy shifts in membrane related core levels with the Manning/Donnan model predictions as a function of external a NaCl and b MgCl2 solution concentration. Error bars represent experimental uncertainty and are the standard deviation of repeated measurements of a minimum of four different locations in each case. Dashed lines are the linear fits of ambient pressure X-ray photoelectron spectroscopy (APXPS) experimental data for external NaCl and MgCl2 solutions with slopes of 0.049±0.004 and 0.026±0.002, respectively. (Brief discussion and further analysis of experimental uncertainties and statistical significance are provided in Supplementary Note 7). Details on Donnan potential calculations using the Manning/Donnan models are given in Supplementary Note 8. The absolute difference between the linear fit of the experimental data and predictions from the analytical model is provided to better capture the comparison between theory and experiment for each electrolyte concentration.