Real-Space Investigation of the Multiple Halogen Bonds by Ultrahigh-Resolution Scanning Probe Microscopy
Result of the Month
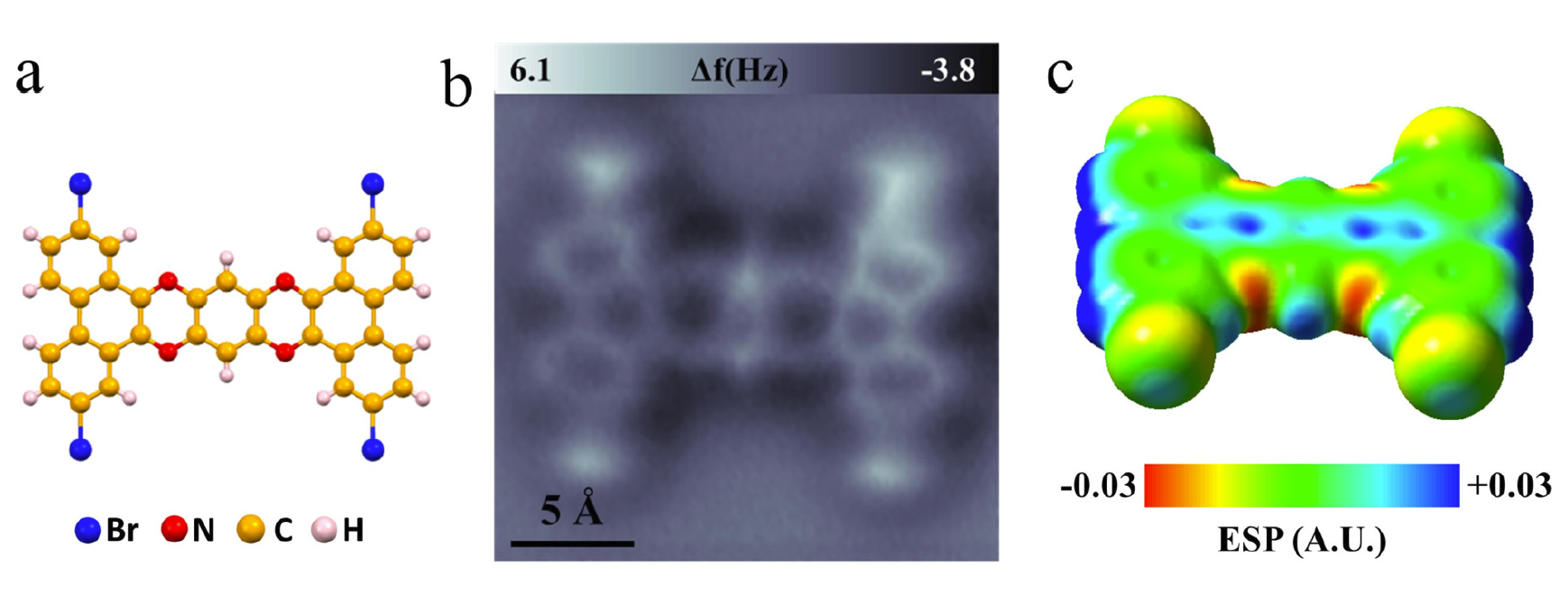
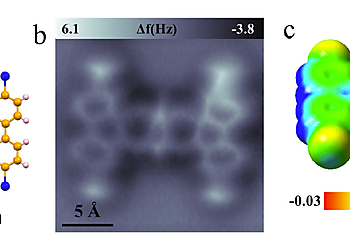
Figure 1. Atomic structure of a single 2-TBQP molecule. (a) Atomic structure of 2-TBQP molecule. (b) Atomic resolution nc-AFM image of a single 2-TBQP molecule on Au(111) acquired with a CO-modified tip, set-point: V = 50 mV, I = 20 pA, tip height Z = -0.1 Å and oscillation amplitude A = 40 pm.
Figure 1 displays atomic structure of a 2-TBQP molecule. The 2-TBQP molecule is a planar polycyclic hydrocarbon with four doping nitrogen atoms (red atoms) and four bromine atom terminals (blue atoms). An atomic resolution nc-AFM image of a single 2-TBQP molecule is acquired with a CO-modified tip. Nine benzene rings are clearly imaged with four bright spots at the terminals, corresponding to the four Br-atoms.
Characterization:
LT-STM/nc-AFM measurements were carried out an integrated scanning probe system consists of Scienta Omicron low-temperature scanning tunneling microscopy (LT-STM) combined with non-contact atomic force microscopy (nc-AFM). The LT-STM images were recorded in both constant current/height mode using AFM tip with functional CO molecule, and bias voltages were applied to the sample. For nc-AFM images, the constant-height mode with AFM CO-tip was used to record the frequency shift (Δf) of the qPlus resonator (sensor frequency f0 ≈ 27000 Hz, Q ≈ 25000). All the measurements were performed at 4.2 K under a base pressure better than 10-11 mbar.
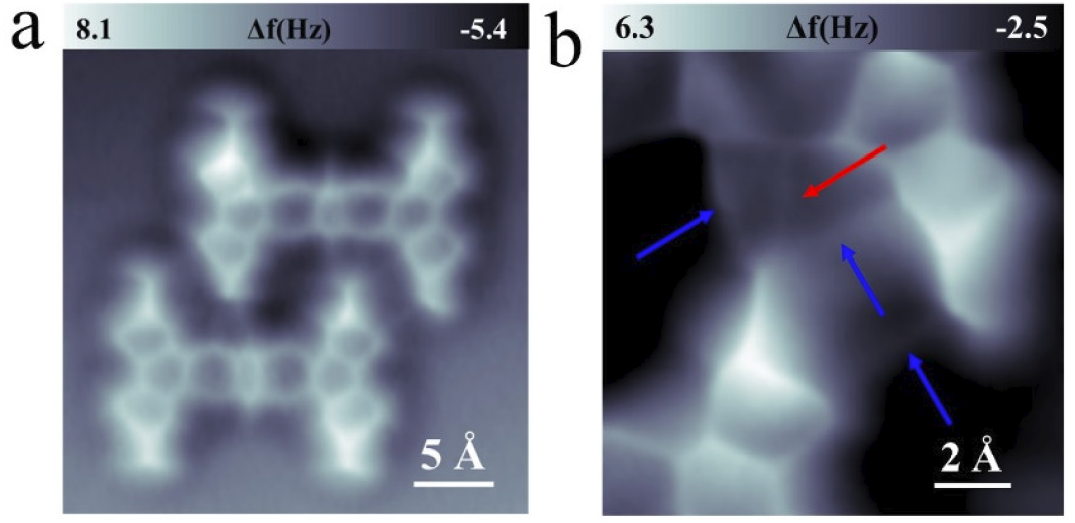
Figure 2. Two types of Br-N halogen bonds. (a) Atomic resolution nc-AFM image of type-1 dimer, set-point: V = 60 mV, I = 20 pA, tip height Z = 0.3 Å and oscillation amplitude A = 40 pm. (b) An enlarged nc-AFM image of type-1 dimer, set-point: V = 60 mV, I = 20 pA, tip height Z = -0.2 Å and oscillation amplitude A = 40 pm.
Figure 2 displays a 2-TBQP dimer. Two Br-atoms are observed to be adjacent to two N-atoms, respectively. An enlarged nc-AFM image in Figure 2b shows that a bright line (labeled by a red arrow) between the adjacent Br-N atoms appears. Three bright lines (labeled by blue arrows) are also observed at the vicinity of the Br-N bright line, which connects three series of Br-H atoms, respectively.
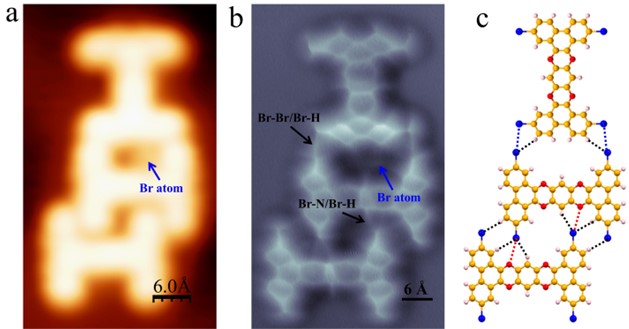
Figure 3. A 2-TBQP trimer stablized by the combination of Br-Br and Br-N halogen bonds. (a) LT-STM image and (b) high-resolution nc-AFM image (c) molecular packing structure of a 2-TBQP trimer on Au(111), set point: a) V = 100 mV and I = 20 pA; b) V= 60 mV, I= 20 pA, tip height Z = -0.1 Å and oscillation amplitude A = 40 pm. The blue arrow indicates a Br atom absorption that is adjacent to one nitrogen atom site, and the black arrows indicate the Br-Br/Br-H and Br-N/Br-H halogen bonds.
Figure 3 displays a 2-TBQP trimer. The trimer forms by the combination of a dimer (stabilized by Br-N/Br-H halogen bonds, as previously discussed) and a third molecule joined via Br-Br/Br-H bonds. The Br-N/Br-H and Br-Br/Br-H halogen bonds are labeled by black arrows. An individual Br-atom (labeled by a blue arrow) adsorbs on the N-atom site of the dimer. We propose that due to steric hindrance arising from individual Br-atom adsorption, a third molecule joins to the dimer via Br-Br/Br-H bonds rather than Br-N/Br-H bonds.
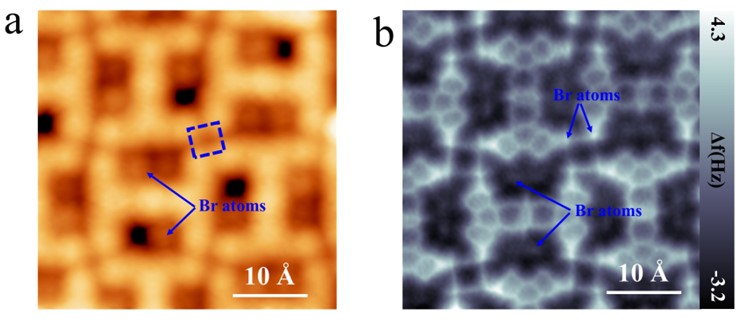
Figure 4. Formation of 2-TBQP tetramer stablized by tetragonal halogen bonds. (a) STM and (b) nc-AFM images of 2-TBQP tetramer on Au(111), set point: a) V = 50 mV and I = 20 pA; b) V=50 mV, I=20 pA, Z=0 Å and oscillation amplitude A = 60 pm; the blue arrows indicate the two kinds of Br-atoms.
Figure 4 displays another self-assembled structure of 2-TBQP tetramer. Figure 4a gives a STM image of the 2-TBQP tetramer. Individual Br-atoms (labeled by blue arrows) adsorb on the periphery of 2-TBQP molecules. Four Br-atoms (labeled by a blue quadrate in Figure 4a) are adjacent, suggesting that the formation of tetragonal Br-Br halogen bonds governs the self-assembled 2-TBQP tetramer. To verify our hypothesis, nc-AFM measurements were carried out. Figure 4b shows a nc-AFM image of the 2-TBQP tetramer, which shows their molecular atomic structure and the real-sapce atom array.
Corresponding author:
Prof. A. T. S. Wee
E-mail: phyweets@nus.edu.sg