Symmetry Breakdown of 4,4″-Diamino-p-Terphenyl on a Cu(111) Surface by Lattice Mismatch
Result of the Month
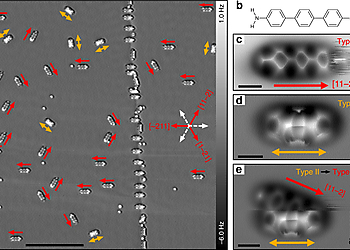
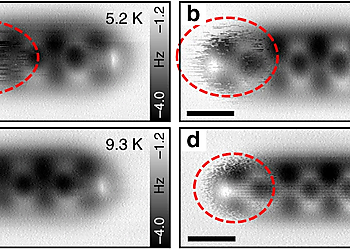
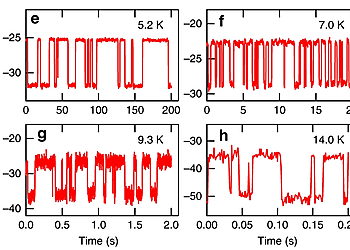
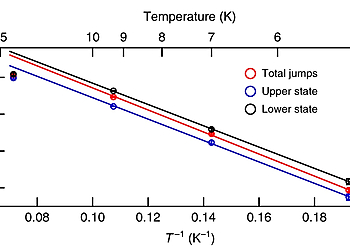
Site-selective functionalization of only one of two identical chemical groups within one molecule is highly challenging, which hinders the production of complex organic macromolecules. Here we demonstrate that adsorption of 4,4″-diamino-p-terphenyl on a metal surface leads to a dissymmetric binding affinity. With low temperature atomic force microscopy, using CO-tip functionalization, we reveal the asymmetric adsorption geometries of 4,4″-diamino-p-terphenyl on Cu(111), while on Au(111) the symmetry is retained. This symmetry breaking on Cu(111) is caused by a lattice mismatch and interactions with the subsurface atomic layer. The dissymmetry results in a change of the binding affinity of one of the amine groups, leading to a non-stationary behavior under the influence of the scanning tip. Finally, we exploit this dissymmetric binding affinity for on-surface self-assembly with 4,4″-diamino-p-terphenyl for side-preferential attachment of 2-triphenylenecarbaldehyde. Our findings provide a new route towards surface-induced dissymmetric activation of a symmetric compound.
AFM Measurements
The measurements were performed with a commercial combined low temperature AFM/STM (Scienta Omicron, Germany). All STM/AFM images were acquired at 5 K (Except for Fig. 2b–d) under ultra-high vacuum (base pressure < 1.0 × 10–10 mbar). For STM imaging, the tip was connected to the ground while the sample was in contact with the bias voltage. For AFM imaging, a small offset gap voltage (a few uV) was used to minimize the tunneling current. The AFM imaging was realized with a force sensor based on the qPlus quartz tuning fork design. Two different force sensors were used—for Fig. 4b, Supplementary Fig. 9a,b: resonance frequency fres ≈ 19.4 kHz, Q-factor ≈ 6300, oscillation amplitude Amp = 94 pm; for all other AFM images: resonance frequency fres ≈ 27.0 kHz, Q-factor > 10,000, oscillation amplitude Amp ≈ 60 pm (the oscillation amplitude for Fig. 1c is 143 pm). A PLL bandwidth of 10 Hz and a scanning speed of 380–630 pm/s were applied to all the AFM images. (Exceptions: scanning speeds are 1750 pm/s, 2000 pm/s, and 1130 pm/s for Supplementary Fig. 1, Fig. 5a, and Supplementary Fig. 9a, respectively.) To achieve submolecular resolution, the tip apex of the metal tip was functionalized with single CO molecules.
Authors:
Qigang Zhong, Daniel Ebeling, Jalmar Tschakert, Yixuan Gao, Deliang Bao, Shixuan Du, Chen Li, Lifeng Chi & André Schirmeisen
Affiliations:
-
Qigang Zhong & Lifeng Chi: Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials and Devices, Soochow University, Suzhou, 215123, P. R. China
-
Daniel Ebeling, Jalmar Tschakert & André Schirmeisen: Institute of Applied Physics, Justus-Liebig University, Heinrich-Buff-Ring 16, 35392, Giessen, Germany
-
Yixuan Gao, Deliang Bao & Shixuan Du: Institute of Physics & University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100190, P. R. China
-
Chen Li: School of Environment and Civil Engineering, Dongguan University of Technology, Dongguan, 523808, P. R. China
Name and email of corresponding author(s):
- Daniel Ebeling
- Shixuan Du
- Lifeng Chi