Selective Triplet Exciton Formation in a Single Molecule
Result of the Month
The exciton formation in organic molecules by charge injection is an essential process in organic light-emitting diodes (OLEDs). According to a simple model based on spin statistics, the injected charges form singlet (S1) excitons and triplet (T1) excitons in a 1:3 ratio1. After the first report of highly efficient phosphorescence OLED2, effective use of T1 has been the primary strategy for increasing the quantum efficiency of OLEDs. Another route to improving OLED energy efficiency is reduction of the operating voltage. Because T1 excitons have lower energy than S1 excitons owing to the exchange interaction, use of the energy difference could—in principle—enable exclusive production of T1 excitons at low OLED operating voltages.
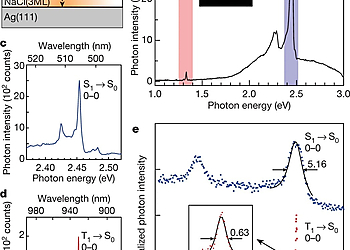
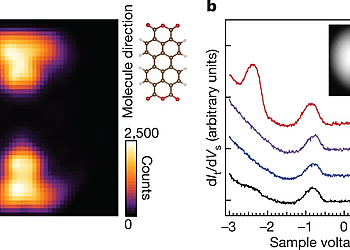
However, a way to achieve such selective and direct formation of these excitons has not yet been established. In this work, we report a single-molecule investigation of electroluminescence using a scanning tunneling microscope (STM)3-5 and demonstrate a simple way for selective formation of T1 excitons that utilizes a charged molecule. A 3,4,9,10-perylenetetracarboxylicdianhydride (PTCDA) molecule adsorbed on a three-monolayer (3ML) NaCl film grown on Ag(111) shows both phosphorescence and fluorescence signals at high applied voltage. In contrast, only phosphorescence occurs at low applied voltage, indicating selective formation of T1 excitons without creating their S1 counterparts. The sample voltage dependence of the phosphorescence, combined with differential conductance measurements, reveals that spin-selective electron removal from a negatively charged PTCDA molecule is the dominant formation mechanism of T1 excitons in this system, which can be rationalized by considering the exchange interaction in the charged molecule. Our findings show that the electron transport process accompanying exciton formation can be controlled by manipulating an electron spin inside a molecule. We anticipate that device designing with consideration of the exchange interaction would realize a novel OLED with a lower operating voltage.
Additional Information
Authors
Kensuke Kimura1,2, Kuniyuki Miwa1,3,5, Hiroshi Imada1*, Miyabi Imai-Imada1,2, Shota Kawahara1, Jun takeya2, Maki Kawai2,4, Michael Galperin3* & Yousoo Kim1*
Institutes
1) Surface and Interface Science Laboratory, RIKEN, Wako, Japan.
2) Department of Advanced Materials Science, Graduate School of Frontier Science, The University of Tokyo, Kashiwa, Japan.
3) Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA, USA.
4) Institute for Molecular Science, National Institutes of Natural Sciences, Okazaki, Japan.
5) Present address: Department of Chemistry, Northwestern University, Evanston, IL, USA.
Name and Email of Corresponding Author
Hiroshi Imada, Michael Galperin & Yousoo Kim
himada@riken.jp ; migalperin@ucsd.edu ; ykim@riken.jp
URL of Institute Web-pages
Kim lab.
RIKEN
Publication(s)
Kimura, K., Miwa, K., Imada, H. et al. Selective triplet exciton formation in a single molecule. Nature 570, 210–213 (2019)
doi:10.1038/s41586-019-1284-2
URL of Journal(s)
References
1) Köhler, A. & Bässler, H. Triplet states in organic semiconductors. Mater. Sci. Eng. R Rep. 66, 71–109 (2009).
2) Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
3) Imada, H. et al. Real-space investigation of energy transfer in heterogeneous molecular dimers. Nature 538, 364–367 (2016).
4) Imada, H. et al. Single-Molecule Investigation of Energy Dynamics in a Coupled Plasmon-Exciton System. Phys. Rev. Lett. 119, 013901 (2017).
5) Miwa, K. et al. Many-Body State Description of Single-Molecule Electroluminescence Driven by a Scanning Tunneling Microscope. Nano Lett. 19, 2803–2811 (2019).