Role of Alkali Cations in Stabilizing Mixed-Cation Perovskites to Thermal Stress and Moisture Conditions
Result of the Month
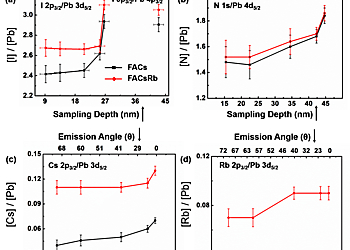
The I/Pb ratio decreases to lower values towards the surface, implying the presence of iodine ion vacancies at the surface. The incorporation of rubidium iodide into the precursor solution has the beneficial effect of improving the material stability by filling iodine ion vacancies, as measured with a higher ratio of I/Pb (towards the expected stoichiometry of 3). The ratio of N/Pb is almost the same for both samples, but shows a downward trend as the emission angle is increased, indicating a smaller amount of organic cation at the surface. HAXPES has enabled us to accurately calculate the chemical distribution of dilute Cs+ and Rb+ alkali-metal cations because of the larger relative sensitivity factor (RSF) of the signals measured for Rb and Cs. For Rb, the measurement of 2p core level at 1805 eV using the Ga Kα source yields a sensitivity ca. 18 times greater than measuring the 3d core level with convention Al Kα source. Also, the sensitivity of Cs 2p measured at Ga Kα is 4.5 greater than that measured using the 3d core level and an Al Kα source. These insights have enabled us to identify the role of Rb in enhancing the stability of these mixed cation perovskites.
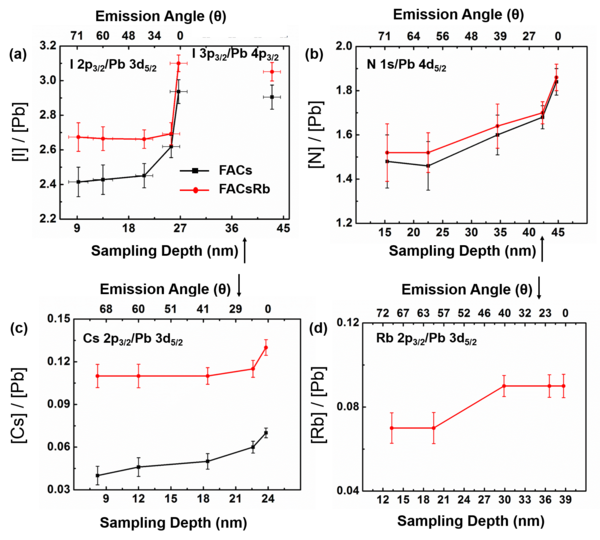
Figure Description: Elemental ratios from HAXPES as a function of estimated sampling depth (bottom x-axis) and electron emission angle (top x-axis) for perovskite solar cell material without (FACs, black) and with (FACsRb) additional rubidium (red): (a) I/Pb, (b) N/Pb, (c) Cs/Pb, and (d) Rb/Pb ratio of the FACsRb sample. The conversion between electron emission angle and sampling depth assumes an IMFP for the numerator signal in each ratio and is thus approximate.
The high-resolution core-level spectra of I 2p, Pb 3d, Cs 2p, Rb 2p were obtained as a function of photoelectron emission angle in order to quantify the chemical composition deeper within the sample. The sampling depth in the material is decreased as the emission angle relative to normal emission is increased according to the following equation where is the inelastic mean free path (obtained from TPP-2M formula) and is the photoelectron emission angle.
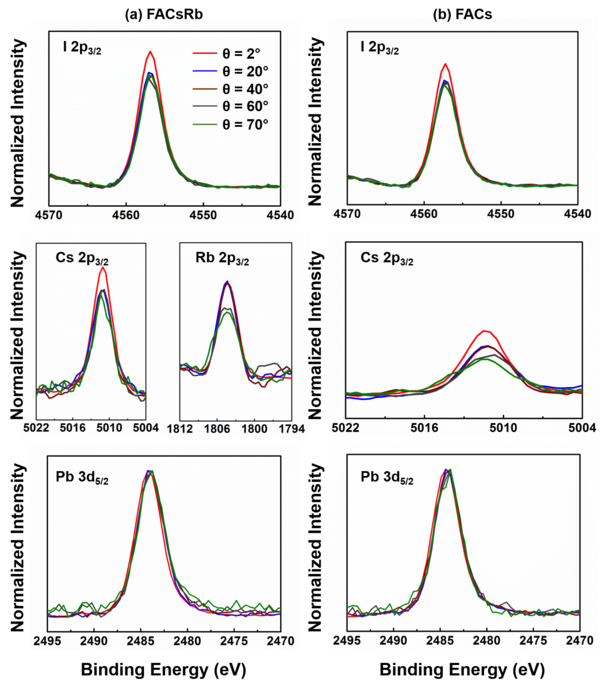
Figure Description: High-resolution I 2p3/2 (top panels), Cs 2p3/2, Rb 2p3/2 (middle panels), and Pb 3d5/2 (bottom panels) core-level spectra of the perovskite material with (FACsRb) (a) and without (FACs) (b) rubidium as a function of electron emission angle (2°, red; 20°, blue; 40°, brown; 60°, grey; and 70°, green) relative to the surface normal, measured using HAXPES. Higher emission angle corresponds to the lower sampling depth, according to eq. S1. The peaks are intensity normalized with respect to Pb 3d5/2.
Method: HAXPES
In this work, the elemental distribution and bulk composition of the multiple-cation perovskite materials were studied through angle-dependent HAXPES measurements with a constant energy X-ray source. The high-throughput HAXPES Lab (Scienta Omicron) is located in the Henry Royce Institute, University of Manchester. The high-energy X-rays are produced by a Ga Kα metal jet X-ray source (Excillum, 9.25 keV) which is monochromated and micro-focused to a 50 μm spot which provides a uniquely high X-ray flux-density. The spectrometer is equipped with an EW-4000 electron energy analyser which can measure photoelectrons with kinetic energy (KE) of up to 12 keV and a wide angular acceptance of up to 60°. The samples were measured using different photoelectron emission angles (θ = 2−70°, with respect to the surface normal) by tilting the samples with respect to the analyser normal. Atomic concentrations were obtained using calculated RSFs for each core level which are explained and listed in the Supporting Information.
Authors:
Suresh Maniyarasu, J. Chun-Ren Ke, Ben F. Spencer, Alex S. Walton, Andrew G. Thomas, and Wendy R. Flavell
Institutes:
-
Andrew G. Thomas − Photon Science Institute, Department of Materials, School of Natural Sciences, and Henry Royce Institute, The University of Manchester, Manchester M13 9PL, U.K.; orcid.org/0000-0002-1900-6686; Email: wendy.flavell@manchester.ac.uk
-
Wendy R. Flavell − Department of Physics and Astronomy, School of Natural Sciences, The University of Manchester, Manchester M13 9PL, U.K.; Photon Science Institute and Henry Royce Institute, The University of Manchester, Manchester M13 9PL, U.K.; orcid.org/0000-0002-2457- 3669; Email: andrew.g.thomas@manchester.ac.uk
-
Suresh Maniyarasu − Department of Physics and Astronomy, School of Natural Sciences, The University of Manchester, Manchester M13 9PL, U.K.; Photon Science Institute, The University of Manchester, Manchester M13 9PL, U.K.; orcid.org/0000-0001-7375-7547
-
J. Chun-Ren Ke − Department of Physics and Astronomy, School of Natural Sciences, The University of Manchester, Manchester M13 9PL, U.K.; Photon Science Institute, The University of Manchester, Manchester M13 9PL, U.K.; orcid.org/0000-0002-6398-1753
-
Ben F. Spencer − Department of Materials, School of Natural Sciences and Henry Royce Institute, The University of Manchester, Manchester M13 9PL, U.K.; orcid.org/0000- 0002-1453-5327
-
Alex S. Walton − Photon Science Institute and Department of Chemistry, School of Natural Sciences, The University of Manchester, Manchester M13 9PL, U.K.; orcid.org/0000-0002-3207-8406
Corresponding Authors:
-
Andrew G. Thomas
Email: wendy.flavell@manchester.ac.uk
-
Wendy R. Flavell
Email: andrew.g.thomas@manchester.ac.uk
Institute Websites:
Photon Science Institute, Department of Materials, School of Natural Sciences, The University of Manchester https://www.psi.manchester.ac.uk/
Department of Physics and Astronomy, School of Natural Sciences, The University of Manchester https://www.physics.manchester.ac.uk/
Henry Royce Institute, The University of Manchester https://www.royce.ac.uk/
Publication:
ACS Appl. Mater. Interfaces 2021, 13, 36, https://doi.org/10.1021/acsami.1c10420