On-Surface Synthesis Guided by Supramolecular Orientation on a Au(111) Surface
Result of the Month
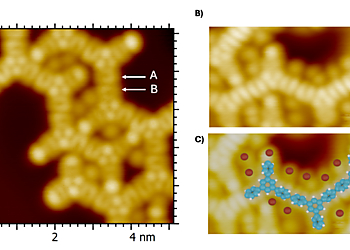
The lines observed in the STM images following thermal annealing at temperatures above 383 K of the initial TBB porous supramolecular network described below in the image 2, correspond to the formation of polymers through an Ullmann-type coupling between TBB molecules. The single protrusions correspond to bromine atoms adsorbed on the surface, which are generated through thermally-induced debromination of the TBB molecules. The formation of unexpected 1D-sawtooth-like polymer lines is clearly governed by the initial supramolecular network, which is centered around an X3-synthon. The High-resolution experiments with the Infinity closed-cycle He STM, without a CO tip, are achieved onto the core of the molecules. The two different orientations of phenyl groups are visible (see arrows A and B).
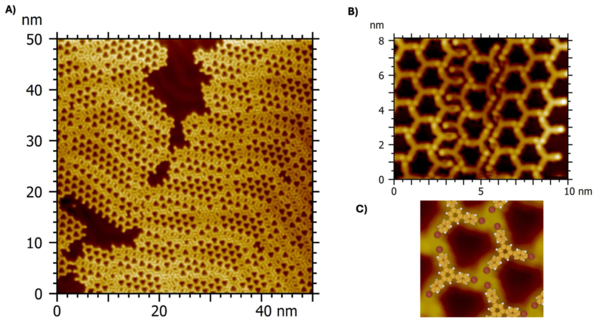
A) A large-scale STM image (50×50 nm2, Vs = -1.37 V, It = 400 pA, 10 K) showing the initial porous supramolecular networks obtained for 0.8 monolayers of 1,3,5-tris(4'-bromophenyl)benzene (TBB) molecule onto an Au(111) surface at 80 K. B) High-resolution STM image (10×8 nm2, Vs = -0.01 V, It = 440 pA, 10 K) of the TBB/Au(111) interface. C) Supramolecular network model superimposed to the STM image.
The initial porous molecular network is formed at 80 K by three TBB molecules that interact through three halogen bonds in a X3-synthon geometry (q1 = 180° and q2 = 120°), as highlighted by the dashed white triangle. The grain boundaries are stabilized by two halogen bonds between two TBB molecules (white arrows). The distance between bromine atoms involved in all halogen bonds is 0.38 ± 0.04 nm.
The thermal activation at 383 K triggers the cleavage of C-Br bonds which induces the generation of radicals with a sufficiently extended lifetime on the Au(111) surface. Due to the X3-synthon arrangement, the phenyl groups carrying a radical only requires half of them to rotate by -30° clockwise, while the remaining half rotates by +30° anticlockwise. In addition, the formation of C-C bonds also requires a translation of approximately half the distance between two bromine atoms.