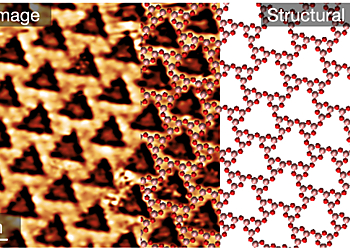
Two-dimensional diboron trioxide crystal composed by boroxol groups
Result of the Month
Diboron trioxide (boria, B2O3) represents a peculiar case. Macroscopic crystallization of boria occurs only under applied pressure, an effect known as crystallization anomaly. Moreover, its two known crystalline phases, B2O3-I and B2O3-II, are possibly made of two different building blocks—regular chains of corner-linked [BO3] triangles in B2O3-I, and [BO4] tetrahedra in B2O3-II — while its vitreous phase shows the presence of yet another structural unit, the so called boroxol [B3O6] group. Because the B2O3 -I phase formation occurs under pressure, Wright (Phys. Chem. Glasses 59, 65–87 (2018)) questions whether B2O3-I is truly the most stable ambient-pressure polymorph or whether another stable ambient-pressure B2O3 crystalline polymorph based on boroxol groups might exist. Ferlat et al. (Nat. Mater.11, 925–929 (2012)) offered a tentative rationalization of this complex scenario and predicted through ab initio calculations the existence of previously unknown microporous crystalline B2O3 polymorphs, but this explanation has not yet been verified experimentally.
Here we report experimental evidence of a 2D B2O3 crystalline polymorph, synthesized on a Pt(111) substrate and composed exclusively by boroxol groups arranged in a honeycomb lattice, confirming the prediction of Ferlat et al. and addressed the question raised by Wright. Through a detailed joint experimental (using STM, LEED, XPS, NEXAFS, and ARPES) and theoretical structural characterization, we demonstrate that 2D boria is an innovative atomically thin material with intriguing structural properties. The boroxol 2D network is nanoporous by construction and constitutes the inorganic rendition of 2D polyphenylene, with the difference that the covalent bonding between benzene rings is replaced by oxygen atoms bridging B3O3 rings. This peculiar linker gives the structure a distinctive mechanical softness—the calculated isotropic stress tensor is one order of magnitude lower than that of graphene—enabling possible tuning of the nanopore size by tensile strain. Moreover, the fabricated 2D boria can cover most of the substrate surface with a mosaic of rotational domains that extend over highly ordered areas of tens of square micrometers, with negligible defect density. We used ab initio numerical simulations to demonstrate that the electronic interaction between the Pt substrate and the 2D boria network was very weak, quantitatively comparable to that occurring between 2D bilayer silica and the same substrate. This finding suggests the possibility of transferring the 2D film to other substrates for applications. Finally, the synthesis of 2D boria makes it possible to extend the investigation of the crystalline-vitreous transition with atomic resolution, and to characterize at the atomic scale the breaking up and reformation of boroxol groups into triangular units upon thermal annealing.